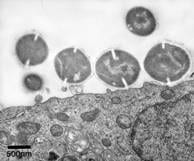
 | High resolution imaging techniques are used to investigate S. pyogenes interactions with human keratinocytes. |
Research in the Caparon laboratory is directed at understanding the interaction of pathogenic Gram-positive bacteria and their human hosts. Of particular interest is Streptococcus pyogenes, the causative agent of diseases including strep throat, scarlet fever, and rheumatic fever.
Signaling interactions are a focus, including how the host environment influences regulation of genes, how the streptococcus influences the signaling responses between various host cells and how the streptococcal cells in one location in a lesion can influence gene regulation in other streptococci located distally in the lesion. As a model of environmental regulation we are characterizing a novel inducible response to oxidative stress that involves no known peroxidase. We have identified a gene that regulates this response and are searching for the effectors of the resistance response.
Our model of how streptococci influence virulence gene regulation in other streptococci is the SpeB cysteine protease, which is expressed in a pattern dependent on cell density in addition to other environmental cues. Following secretion, the proprotease must fold in the extracellular spaces and adopt a conformation where it can undergo autoactivation. We have identified several genes that regulate protease expression.
In analyzing how streptococci manipulate the signaling responses of host cells, we have identified “Cytolysin-Mediated Translocation,” a novel pathway for the injection of streptococcal proteins directly into the cytosol of host cells. At least one of the translocated proteins has characteristics of a eukaryotic signal transduction factor. This pathway may be widespread among Gram-positive pathogens and be the functional equivalent of a Type III secretion pathway.
Another interest of the lab is the localization and coordination of protein secretion and folding in Gram-positive cocci. An investigation of the subcellular organization of the general secretory machinery as well as surface anchored chaperones that assist in the biogenesis of secreted proteins in S. pyogenes revealed the “ExPortal,” a distinct microdomain in the bacterial membrane.